Abstract
Background: Treatment with the highly selective Bruton tyrosine kinase inhibitor (BTKi) acalabrutinib (A), administered with or without obinutuzumab (O), has demonstrated significant progression-free survival (PFS) benefits compared with chemoimmunotherapy in patients (pts) with treatment-naive (TN) chronic lymphocytic leukemia (CLL), including those with higher-risk genomic characteristics (ELEVATE-TN; Sharman et al. Leukemia. 2022;36:1171-5); significantly better PFS and numerically better overall survival (OS) outcomes were also seen with A+O vs A monotherapy, albeit with some additional toxicity observed. However, a better understanding of the impact of adding O to A in pts with TN CLL is needed, particularly because adding rituximab to ibrutinib did not improve outcomes in the Alliance A041202 trial (Woyach et al. N Engl J Med. 2018;379:2517-28). To assess the contributory effects of adding O to A in first-line CLL, we performed a pooled analysis of 2 clinical studies (ELEVATE-TN and CL-003) to compare PFS and OS for A+O vs A monotherapy in pts with TN CLL by prognostic factors within each genomic characteristic.
Methods: In this retrospective analysis, PFS and OS outcomes were compared between the A+O and A arms by prognostic factors (age, bulky disease, CLL International Prognostic Index [CLL-IPI] score and beta-2 microglobulin (B2M) levels at baseline) in pts with and without the selected genomic characteristics. Del(17p) and/or TP53 mutation [del(17p)/TP53m], unmutated IGHV (uIGHV), and complex karyotype (CK, ≥3 chromosomal abnormalities) were considered higher-risk genomic features in this analysis and analyzed separately. Lower-risk genomic features (absence of del(17p)/TP53m, mutated IGHV, and absence of CK) also were analyzed separately. The impact of co-mutation was not assessed.
Results: The pooled analysis included 376 pts (A+O, n=197; A monotherapy, n=179). Overall, 28 (14%) A+O and 24 (13%) A pts had del(17p)/TP53m, 112 (57%) and 118 (66%) had uIGHV, and 35 (18%) and 32 (18%) had CK.
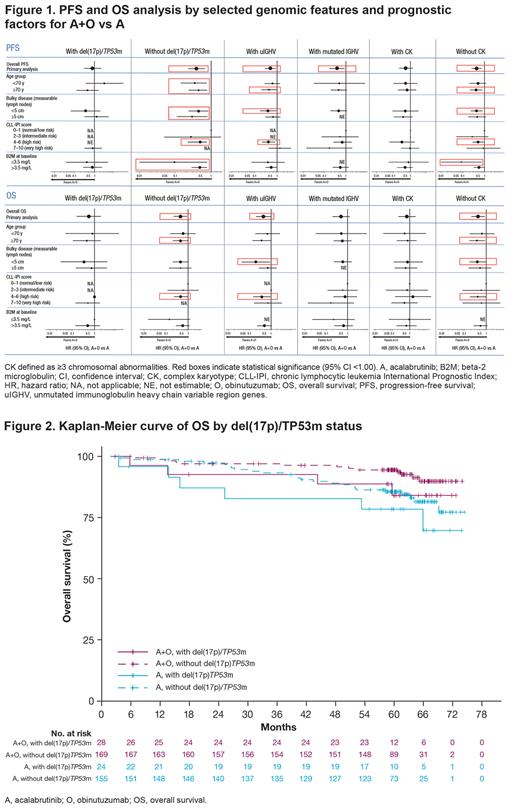
PFS improved with A+O vs A in pts with uIGHV (hazard ratio [HR] 0.51; 95% confidence interval [CI] 0.29-0.89). The HRs for the PFS comparisons between the A+O and A arms were 0.82 (95% CI 0.31-2.18) and 0.56 (95% CI 0.21-1.52) for pts with del(17p)/TP53m and CK, respectively. PFS improved with A+O vs A in pts without del(17p)/TP53m (HR 0.38; 95% CI 0.23-0.65), pts with mutated IGHV (HR 0.39; 95% CI 0.17-0.89), and pts with no CK abnormality (HR 0.43; 95% CI 0.25-0.72). PFS benefits of A+O vs A by subgroup are shown in Figure 1.
OS was improved with A+O vs A in pts with uIGHV (HR 0.44; 95% CI 0.20-0.97). No significant improvement was noted in pts with del(17p)/TP53m (HR 0.55; 95% CI 0.16-1.95) (Figures 1, 2) or in pts with CK (HR 0.69; 95% CI 0.21-2.27). OS was improved in pts without del(17p)/TP53m (HR 0.44; 95% CI 0.23-0.87) and in pts with no CK abnormality (HR 0.41; 95% CI 0.21-0.82); the HR for the OS comparison between the A+O and A arms in pts with mutated IGHV was 0.49 (95% CI 0.20-1.22). OS benefits of A+O vs A by subgroup are shown in Figure 1.
Conclusions: This retrospective pooled analysis of clinical trial data in 376 pts demonstrated the benefit of adding O to A monotherapy across genomic subgroups, particularly in pts with uIGHV or without del(17p)/TP53m or CK abnormalities. The benefit of adding O was not as evident in pts with del(17p)/TP53m or CK; however, the sample size was limited in these subgroups. Thus, it is unclear whether the addition of O imparts additional benefit beyond that of a BTKi alone to change the natural course of disease in TP53-aberrant pt populations. Overall, this analysis sheds light on the CLL pt populations most likely to benefit from the addition of O to A across various genomic and clinical prognostic subgroups. Novel combination strategies might still be needed to further improve outcomes in pts with del(17p)/TP53m.
Disclosures
Davids:Research to Practice: Honoraria; Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees; Eli Lilly and Company: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy; TG Therapeutics: Consultancy, Research Funding; Novartis: Research Funding; Merck: Consultancy; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel expenses, Research Funding; Verastem: Consultancy, Research Funding; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Ono Pharmaceuticals: Consultancy; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Ascentage Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Sharman:TG Therapeutics: Consultancy, Research Funding; Merck: Consultancy; Lilly: Consultancy, Honoraria, Research Funding; Araris Biotech AG: Consultancy, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics LLC, an AbbVie Company: Honoraria; ADC Therapeutics: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Beigene: Consultancy, Honoraria, Research Funding; Genentech: Consultancy; BMS: Consultancy, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding. Eyre:Secura Bio: Membership on an entity's Board of Directors or advisory committees; Medscape: Speakers Bureau; PeerView: Speakers Bureau; LOXO Lilly: Membership on an entity's Board of Directors or advisory committees, Other, Speakers Bureau; Incyte: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; KITE: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BeiGene: Research Funding; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Woyach:ArQule: Consultancy; Genentech: Consultancy; Newave: Consultancy; Janssen: Consultancy; AbbVie: Consultancy, Research Funding; MorphoSys: Consultancy, Research Funding; BeiGene: Consultancy; Loxo@Lilly: Research Funding; AstraZeneca: Consultancy; Schrodinger: Research Funding; Karyopharm Therapeutics: Research Funding; Pharmacyclics: Consultancy. de Miranda:AstraZeneca: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Shahkarami:AstraZeneca: Current Employment. Butturini:AstraZeneca: Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months. Emeribe:AstraZeneca: Current Employment. Byrd:Kura Oncology, Inc: Consultancy; Vincerx Pharma: Current equity holder in publicly-traded company; TG Therapeutics: Honoraria; AstraZeneca: Consultancy; Syndax: Consultancy; Pharmacyclics LLC: Honoraria, Research Funding; Xencor, Inc: Research Funding; Novartis: Consultancy, Honoraria; Janssen Pharmaceuticals, Inc.: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal